The Nature of Covalent Bonding 8.2 Answer Key
Single double and triple covalent bonds depend on the number of pairs of electrons shared between two atoms. Stable electron covalent shared single.
The Nature Of Covalent Bonding 8 2
Two atoms held together by sharing one pair of electrons.

. For example each hydrogen atom has one electron. Most atoms share electrons until they have a. 82 The Nature of Covalent Bonding 17.
5-8-George Graybill 2007-09-01 You dont have to be a rocket scientist to understand matter and energy with our Physical Science 3-book BUNDLE. Chemistry 12th Edition answers to Chapter 8 - Covalent Bonding - 82 The Nature of Covalent Bonding - 82 Lesson Check - Page 238 14 including work step by step written by community members like you. Orbital to form the following electron configuration.
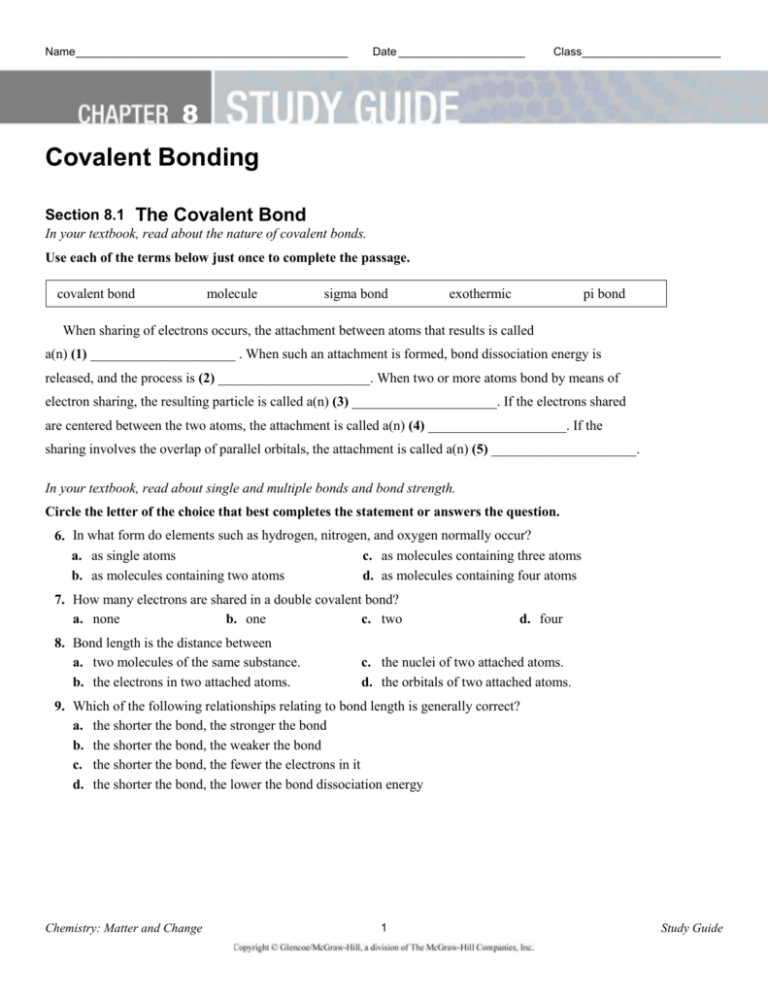
Electrons is promoted to the vacant 2. General Concepts - Part 1Chapter 8 Covalent Bonding Worksheet Section 82 The Nature of Covalent Bonding In ionic bonding atoms transfer electrons to achieve noble gas configuration. Two atoms held together by sharing a pair of electrons are joined by this.
In covalent bonding atoms share electrons to achieve noble gas configuration. Memorize flashcards and build a practice test to quiz yourself before your exam. Chapter 82 The Nature of Covalent Bonding.
A typical carboncarbon single bond has a bond. The formation of four bonds by carbon can be explained by the fact that one of carbons 2. Unit 8 Section Review Answer Key Author.
Atoms form double or triple covalent bonds if they can attain a noble gas structure by doing so. Covalent Bonding Worksheet Answer KeyPart 1Chapter 8 Covalent Bonding Worksheet Section 82 The Nature of Covalent Bonding In ionic bonding atoms transfer electrons to achieve noble gas configuration. In forming covalent bonds electron sharing usually occurs so that atoms attain the electron configurations of noble gases.
Represents the covalent bonds as dashes and shows the arrangement of covalently bonded atoms. Start studying the 82 The Nature of Covalent Bonding Section Assesment flashcards containing study terms like What electron configurations do atoms usually achieve by sharing electrons to form covalent bonds How is an electron dot structure used to represent a covalent bond When are 2. A typical carboncarbon single bond has a bond dissociation energy of 347 Page 1024.
A covalent bond in which one atom contributes both bonding electrons Polyatomic ion a tightly bound group of atoms that has a positive or negative charge and behaves as a unit. The Nature Of Covalent Bonding 8 2 Worksheet Answers 82 The Nature of Covalent Bonding 8 In covalent bonds electron sharing usually occurs so that atoms attain the electron configurations of noble gases. Up to 24 cash back Section Review 82 Part A Completion 1.
82 The Nature of Covalent Bonding 82 The Nature of Covalent Bonding 55 A large bond dissociation energy corresponds to a strong covalent bond. The octet rule in covalent Bonding Covalent compounds are most stable when each atom has eight electrons. 82 The Nature of Covalent Bonding Pearson Accelerated Chemistry Chapter 8.
But a pair of hydrogen atoms share these two electrons when they form a covalent bond in a hydrogen molecule. Students discover what matter is with Properties of Matter. A pair of valence electrons that is not shared between atoms lonenon-bonding pair double covalent bond.
Chemistry 12th Edition answers to Chapter 8 - Covalent Bonding - 82 The Nature of Covalent Bonding - 82 Lesson Check - Page 238 18 including work step by step written by community members like you. Nicolette Kimball Created Date. But a pair of hydrogen atoms share these two electrons when they form a covalent bond in a the.
242 Chapter 8 Covalent Bonding Single Covalent Bonds When only one pair of electrons is shared such as in a hydrogen molecule it is a single covalent bond. Download Free 8 Covalent Bond Practice Problems Answers Key. Polar covalent bonds nonpolar covalent bonds Part D Questions and Problems 22.
Download File PDF 82 The Nature Of Covalent Bonding Answer Key 82 The Nature Of Covalent Bonding Answer Key Thank you utterly much for downloading 82 the nature of covalent bonding answer keyMaybe you have knowledge that people have see numerous times for their favorite books later than this 82 the nature of covalent bonding answer key but stop. The shared electron pair is often referred to as the bonding pair. This electron promotion requires only a small.
In covalent bonding atoms share electrons to achieve noble gas configuration. 81 Molecular Compounds 82 The Nature of Covalent Bonding 83 Bonding Theories 84 Polar Bonds and Molecules. For example each hydrogen atom has one electron.
Most atoms share electrons Page 935. The Nature of Covalent Bonding Ch 8 section 02 Nature of Covalent Bonding PART 2 Video Answer KEY Ch 8 section 02 The Nature of Covalent Bonding PART 1 lecture Ch 8 section 02 Nature of Covalent. 8 2 The Nature Of Covalent Bonding Key Pearson 15 PDF 8 2 The Nature Of Covalent Bonding Key Pearson The Nature of Matter Big Book Gr.
Structural formula Single covalent bond polyatomic ion bond dissociation energy coordinate covalent bond bond dissociation energy A tightly bound group of atoms that has a positive or negative charge and behaves as a unit. In covalent bonds electron sharing usually occurs so that atoms attain the electron configurations of noble gases. Section Review Answer Key 82 The Nature of Covalent Bonding 8 82 The Nature of Covalent Bonding 82 The Nature of Covalent Bonding 55 A large bond dissociation energy corresponds to a strong covalent bond.
Covalent Bonding Answer Key BingNature of Covalent Bonding - 82 Lesson Check - Page 238 19 including work step by step written by community members like you. For a hydrogen molecule shown in Figure 84 each covalently bonded atom equally attracts the pair of shared electrons. The energy required to break the bond.
Copyright Pearson Education Inc or its affiliates. Chemistry 12th Edition answers to Chapter 8 - Covalent Bonding - 82 The Nature of Covalent Bonding - 82 Lesson Check - Page 238 16 including work step by step written by community members like you. Terms in this set 31 Bond dissociation energy.
Chemistry 12th Edition answers to Chapter 8 - Covalent Bonding - 82 The Nature of Covalent Bonding - 82 Lesson Check - Page 238 19 including work step by step written by community members like you. Each hydrogen atom thus attains the electron configuration.

Section Review The Nature Ofcovalent Bonding

No comments for "The Nature of Covalent Bonding 8.2 Answer Key"
Post a Comment